If you’re keen on understanding expansion work in thermodynamics, this is the definitive guide for you. We’ll break down each aspect of this key concept, from its basic definition to its practical applications.
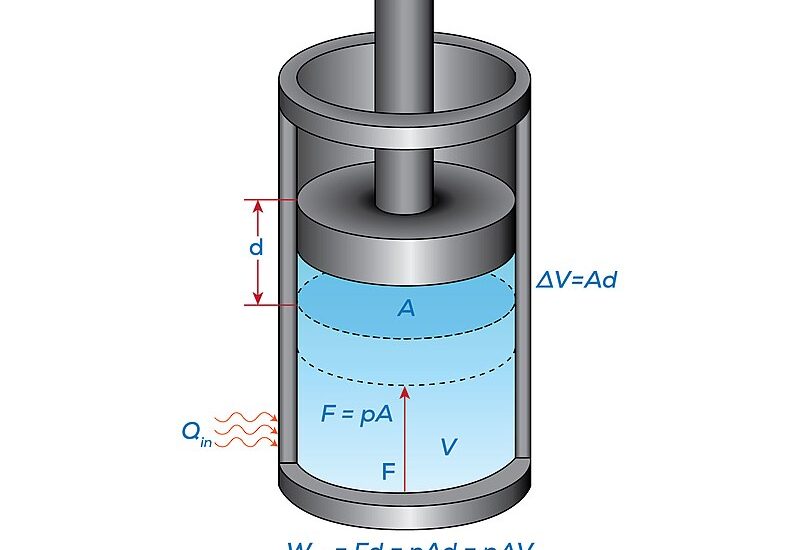
Table of Contents
What is Expansion Work in Thermodynamics?
At its core, expansion work in thermodynamics is the work done by a system (often a gas) when it undergoes a volume change in the presence of an external force, usually pressure.
This might sound complicated, but it’s like blowing up a balloon. When you blow air into a balloon, you’re doing work against the balloon’s material and atmospheric pressure to expand its volume. That’s expansion work.
Why is it important? Well, this type of work is essential for understanding how energy is transferred in various systems, which is a foundational concept in thermodynamics. Expansion work is a way to quantify this energy transfer in numerical terms.
Types of Expansion Work in Thermodynamics
Expansion work can occur under different conditions, resulting in various types:
Isobaric Expansion: This occurs at constant pressure. Imagine blowing up a balloon without any resistance other than the material of the balloon itself.
Isothermal Expansion: Here, the temperature remains constant throughout the process. Think of a tire inflating on a hot day but not getting any hotter.
Adiabatic Expansion: No heat is exchanged with the surroundings in this type of expansion. It’s like a quick release of air from a tire, where the air inside doesn’t have time to absorb or release heat.
Each of these types has specific mathematical equations that describe the work done, which leads us to our next section.
Check out these other related articles…
Adjusting TXV for Subcooling: The Ultimate How-To Guide
Car Expansion Valve Adjustment: In 5 Easy Steps
What is TXV: Your Comprehensive 411 Guide
Expansion Valve Pressure Drop: What You Need to Know
Expansion Valve Test: How to Perform in 7 Easy Steps
Where is the AC Expansion Valve Located? Comprehensive Guide
Calculating Expansion Work
To calculate expansion work, you’ll need different equations for different types of expansion.
Isobaric: The formula is straightforward: W = PΔV. Here, W represents work, P stands for constant pressure, and ΔV is the change in volume. It’s a simple multiplication.
Isothermal: Things get slightly complicated. The formula is W = nRT ln(Vf/Vi). In this equation, n stands for the number of moles of the gas, R is the universal gas constant, T is the temperature in Kelvin, and Vf and Vi are the final and initial volumes, respectively.
Adiabatic: The formula for adiabatic work is W = Cv (Tf - Ti). In this equation, Cv is the heat capacity at constant volume, and Tf and Ti are the final and initial temperatures, respectively.
Practical Applications
Expansion work is not just an abstract concept; it has real-world applications.
Engineering: Engineers use these principles to design more efficient engines and machinery.
Physics: In scientific research, understanding expansion work can help to explain various natural phenomena.
Chemistry: In chemical reactions involving gases, calculating expansion work can be critical.
Daily Life: Items like refrigerators, air conditioners, and even your car engine work based on these principles.
Check out this comprehensive resource on all things expansion valve.
FAQs on Expansion Work
Here we address some of the frequently asked questions for a more rounded understanding:
What are the units of expansion work? The most common units are Joules (J) or liters-atmospheres (L·atm).
Is expansion work always positive? No, it can be negative if the system is compressed rather than expanded. In this case, work is done on the system rather than by it.
Does expansion work occur only in gases? While it’s more commonly described in gases due to their more substantial volume changes, expansion work can also occur in liquids and solids, albeit rarely.