Understanding the expansion process in thermodynamics is crucial for anyone studying or working in fields related to energy systems. This step-by-step guide aims to break down this complex subject into easily understandable parts.
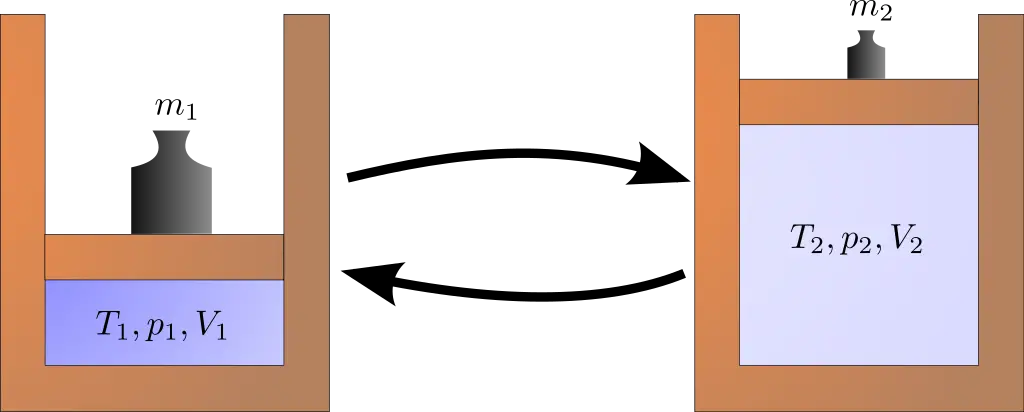
Table of Contents
What is Thermodynamics?
Thermodynamics is a branch of physics that delves into the behavior of energy and matter under different conditions. It provides a framework for understanding how substances interact in terms of heat, work, and energy transformations. The focus is on systems at equilibrium, which means they are not undergoing any net changes over time.
Key Concepts in Thermodynamics
Before discussing the expansion process, it’s imperative to understand certain key terms:
Entropy: A measure of disorder or randomness in a system. It helps predict the direction of thermodynamic processes.
Enthalpy: A property that accounts for heat transfer during constant pressure processes.
Internal Energy: The total energy within a system, consisting of the kinetic and potential energy of its particles.
Laws of Thermodynamics: Four fundamental laws (Zeroth, First, Second, and Third) that govern all thermodynamic processes.
What is the Expansion Process?
The expansion process in thermodynamics pertains to a specific change state in which a fluid, usually a gas or vapor, expands, altering its volume and other state properties. This usually involves doing work on the surroundings.
Expansion can occur under various conditions such as constant temperature (isothermal), no heat exchange (adiabatic), or constant pressure (isobaric).
Types of Expansion Processes
Understanding the different types of expansion processes is crucial:
Isobaric Expansion: Here, the pressure remains constant. Work is done, but the temperature may change.
Isothermal Expansion: Occurs at a constant temperature. Heat from the surroundings is necessary to maintain the temperature.
Adiabatic Expansion: In this type, no heat enters or leaves the system. As a result, work is done at the expense of internal energy.
Polytropic Expansion: A more general case in which the process follows a mathematical relationship between pressure, volume, and temperature.
Check out these other related articles…
Car Expansion Valve Adjustment: In 5 Easy Steps
What is TXV: Your Comprehensive 411 Guide
Expansion Valve Pressure Drop: What You Need to Know
Expansion Valve Test: How to Perform in 7 Easy Steps
Where is the AC Expansion Valve Located? Comprehensive Guide
How Does Expansion Work?
In an expansion process, a fluid changes its volume while doing work on its surroundings. For instance, when a gas expands in a piston, it pushes the piston upwards.
Depending on the type of expansion process, various factors like volume, pressure, and temperature change in a calculable manner. Equations like the Ideal Gas Law or specific heat capacities may be used to quantify these changes.
Applications of the Expansion Process in Thermodynamics
The principles of the expansion process in thermodynamics find applications in a multitude of areas:
Internal Combustion Engines: Gas expands to move pistons, driving a vehicle.
Refrigeration Cycles: Expansion valves in refrigerators facilitate cooling by allowing refrigerant to expand.
Natural Phenomena: Expanding and contracting air masses leads to weather patterns.
Industrial Systems: Many manufacturing processes rely on the controlled expansion of gases or liquids.